Nationwide Drug Recall Affects Millions Managing ADHD
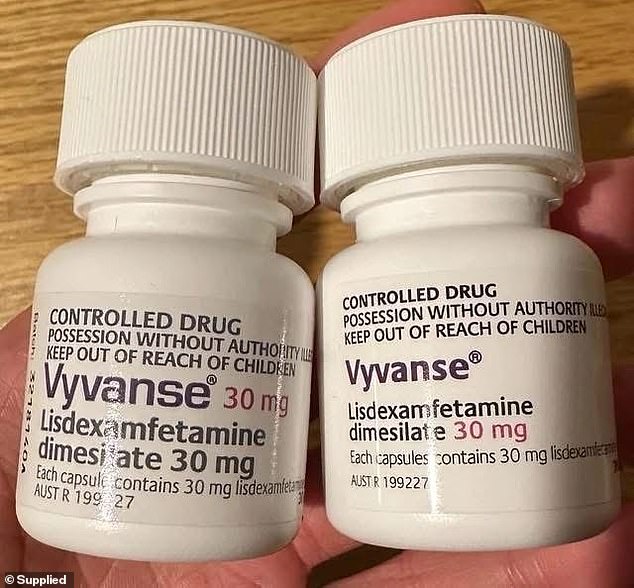
A significant drug recall is underway in the United States, potentially impacting millions of individuals who rely on medication to manage Attention Deficit Hyperactivity Disorder (ADHD). Sun Pharmaceutical Industries has initiated a voluntary recall of several batches of lisdexamfetamine dimesylate capsules, a widely prescribed generic form of ADHD treatment for patients aged six and older. This medication is commonly known by brand names such as Vyvanse and Arynta.
The U.S. Food and Drug Administration (FDA) has classified this action as a Class II recall. This designation signifies that while the product's use "may cause temporary or medically reversible adverse health consequences," the risk of serious harm is considered to be remote.
The Reason Behind the Recall
The recall was triggered by a lapse in laboratory testing procedures. Specifically, the affected batches of medication failed to dissolve properly during mandated quality control tests. This critical defect has raised concerns about how the drug might be absorbed in the body. If the capsules do not dissolve as intended, patients could potentially receive a lower dose than prescribed, leading to reduced therapeutic effectiveness. This could, in turn, result in a worsening of ADHD symptoms, including increased fatigue and difficulties with concentration.

Rising Prescriptions for ADHD Medications
The recall comes at a time when prescriptions for lisdexamfetamine dimesylate have seen a substantial increase across the US in recent years. Data indicates that between 2012 and 2023, overall stimulant prescriptions for ADHD grew by approximately 60 percent. In 2023 alone, lisdexamfetamine accounted for roughly 19 percent of all stimulant prescriptions issued.
More than nine million prescriptions for this medication were dispensed in 2023. This surge in prescriptions is partly attributed to an improved understanding and a rise in ADHD diagnoses, particularly among adults and women, as awareness and screening efforts have advanced. The widespread adoption of telehealth services has also played a role, making it significantly easier for individuals to access ADHD evaluations and obtain prescriptions without the need for in-person visits.
Details of the Recalled Batches
The recall specifically affects 100-count bottles of lisdexamfetamine dimesylate capsules in strengths ranging from 10 mg to 70 mg. The affected products have expiration dates spanning from February 2026 to May 2026. These capsules were manufactured by Ohm Laboratories, Sun's manufacturing division, which is based in New Brunswick, New Jersey.
Recalled Batch Numbers and Expiration Dates:
- 10 mg:
- AD42468 (exp. 2/28/2026)
- AD48705 (exp. 4/30/2026)
- 20 mg:
- AD42469 (exp. 2/28/2026)
- AD48707 (exp. 4/30/2026)
- 30 mg:
- AD42470 (exp. 2/28/2026)
- AD48708 (exp. 4/30/2026)
- 40 mg:
- AD48709 (exp. 4/30/2026)
- AD50894 (exp. 5/31/2026)
- 50 mg:
- AD48710 (exp. 4/30/2026)
- AD50895 (exp. 5/31/2026)
- 60 mg:
- AD48711 (exp. 4/30/2026)
- AD50896 (exp. 5/31/2026)
- 70 mg:
- AD48712 (exp. 4/30/2026)
- AD50898 (exp. 5/31/2026)
Guidance for Patients
Patients who are currently taking lisdexamfetamine dimesylate and suspect they may have medication from an affected batch are strongly advised not to discontinue their treatment abruptly without first consulting a healthcare provider. Suddenly stopping ADHD medication can lead to withdrawal symptoms and a significant exacerbation of ADHD symptoms.
The FDA is urging patients to reach out to their doctor or pharmacist for further guidance. Healthcare professionals can assess individual situations and arrange for safe and appropriate replacement medication if necessary. For more detailed information regarding this recall, patients can visit the FDA's official recall page or contact Sun Pharmaceutical Industries directly.
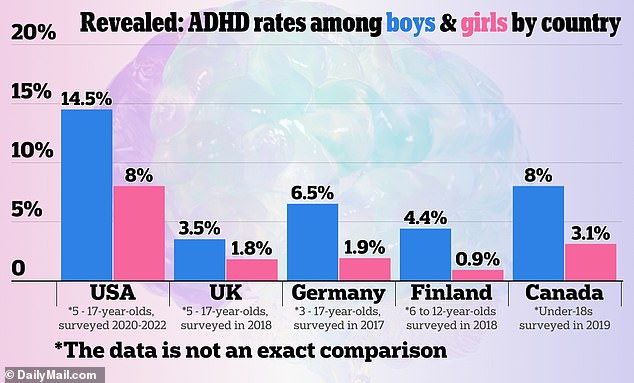
Understanding ADHD and its Treatments
It is estimated that around 22 million Americans have ADHD. Of these, just over half are prescribed medication to help manage their symptoms, which can manifest as impulsiveness, disorganization, and significant difficulties with focusing and maintaining attention.
ADHD medications are broadly categorised into two main types: stimulants and non-stimulants.
- Stimulant Medications: These are the most commonly prescribed for ADHD. They work by enhancing the transmission of dopamine in the brain, a neurotransmitter that plays a crucial role in mood, motivation, and movement. Examples include methylphenidate-based drugs and amphetamine-based medications like lisdexamfetamine dimesylate.
- Non-Stimulant Medications: These are often considered when stimulants are not effective or are not well-tolerated by the patient. Non-stimulants work by improving the transmission of norepinephrine, a hormone that aids in alertness and focus. Examples include atomoxetine, clonidine, and guanfacine.
Lisdexamfetamine dimesylate is a prodrug, meaning it is inactive until it is metabolised by the body into its active form, dextroamphetamine. This pharmacological design is intended to provide a smoother, longer-lasting therapeutic effect and to reduce the potential for misuse compared to some other stimulant medications. Some of the most well-known brand names associated with ADHD medications include Adderall, Ritalin, Vyvanse, Focalin, Concerta, and Daytrana.
The exact causes of ADHD are not fully understood, but research suggests a strong genetic component, as the condition tends to run in families.


No comments:
Post a Comment