According Arrhenius : Acid is a substance which when dissolved in water can produce H + ions. Due to the excess of H + ions then the water with acid substance called acid solution. A base is a substance which when dissolved in water can generate OH- ions. Due to excess OH- ions then the water with base Substances referred to as base solution.
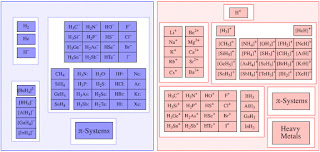
What if an electrolyte substance is dissolved into water, then the electrolyte dye molecules will break into smaller particles called ions. This process is called ionization. Some of positively charged ions, while the other negatively charged ions. The number of positive charge equal to the amount of negative charge. This is because the molecules initially a neutral molecule so that the total amount of charge on the ion is also neutral
Electrical conductivity of the electrolyte solution is due to the presence of these ions. Substance Non-electrolytes can not menghantarakan electric current because there are no ions. The existence of the electrolyte substance in solution will affect the boiling point and freezing point of the solution, therefore increasing the particle in solution (ion).


No comments:
Post a Comment